Hydrogen Production
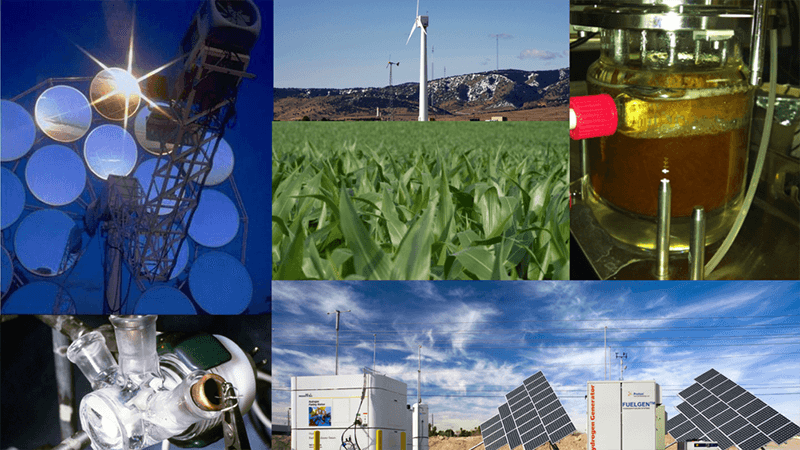
Hydrogen can be produced using diverse, domestic resources. Fossil fuels, such as natural gas and coal, can be converted to produce hydrogen, and the use of carbon capture, utilization, and storage can reduce the carbon footprint of these processes. Hydrogen can also be produced from low carbon and renewable resources, including biomass grown from non-food crops and splitting water using electricity from wind, solar, geothermal, nuclear, and hydroelectric. This diversity of potential supply sources is an important reason why hydrogen is such a promising energy carrier.
Hydrogen can be produced at large central plants, at medium scale semi central plants, or in small distributed units located at or very near the point of use, such as at refueling stations or stationary power sites.
One kg of hydrogen has the same energy content as one gallon of gasoline, on a lower heating value basis.
How Is Hydrogen Produced?
Researchers are developing a wide range of technologies to economically produce hydrogen from a variety of resources in environmentally friendly ways.
Natural Gas Reforming
Hydrogen can be produced from natural gas using high-temperature steam. This process, called steam methane reforming, accounts for about 95% of the hydrogen used today in the United States. Another method, called partial oxidation, produces hydrogen by combusting methane in air. Both steam reforming and partial oxidation produce a synthesis gas or syn gas, which is then reacted with additional steam to produce a higher hydrogen content gas stream.
Gasification
Gasification is a process in which coal or biomass is converted into gaseous components by applying heat under pressure and in the presence of air/oxygen and steam. A subsequent series of chemical reactions produces syn gas, which is then reacted with steam to produce a gas stream with an increased hydrogen concentration that then can be separated and purified. With carbon capture and storage, hydrogen can be produced directly from coal with near-zero greenhouse gas emissions. Since growing biomass consumes carbon dioxide from the atmosphere, producing hydrogen through biomass gasification results in near-zero net greenhouse gas emissions without carbon capture and storage.
Renewable Liquid Reforming
Biomass can also be processed to make renewable liquid fuels, such as ethanol or bio-oil, which are relatively convenient to transport and can be reacted with high- temperature steam to produce hydrogen at or near the point of use. Researchers are also exploring a variation of this technology known as aqueous-phase reforming.
Electrolysis
Electrolysis uses an electric current to split water into hydrogen and oxygen. The electricity can be generated in several ways. However, to minimize greenhouse gas emissions, electricity generation using renewable energy technologies (such as wind, solar, geothermal, and hydroelectric power), nuclear energy, or natural gas and coal with carbon capture, utilization, and storage are preferred.
High-Temperature Electrolysis
Heat from industrial processes such as a nuclear reactor can be used to improve the efficiency of electrolysis to produce hydrogen. By increasing the temperature of the water, less electricity is required to split it into hydrogen and oxygen, which reduces the total energy required.
Solar Thermochemical Water-Splitting (STCH)
Another water-splitting method uses high temperatures generated by solar concentrators (mirrors that focus and intensify sunlight) to drive a series of chemical reactions to split water into hydrogen and oxygen. All of the intermediate process chemicals are recycled within the process.
Photoelectrochemical (PEC)
Hydrogen can be produced directly from water using sunlight and a special class of semiconductor materials. These highly specialized semiconductors absorb sunlight and use the light energy to directly split water molecules into hydrogen and oxygen.
Biological
Certain microbes, such as green algae and cyanobacteria, produce hydrogen by splitting water in the presence of sunlight as a byproduct of their natural metabolic processes. Other microbes can extract hydrogen directly from biomass.
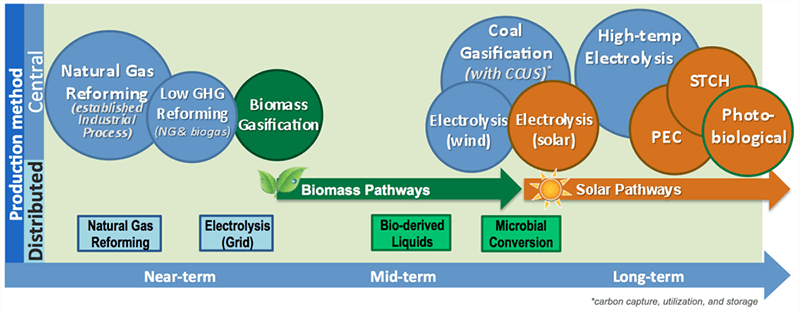
What Are the Challenges?
The greatest challenge for hydrogen production, particularly from renewable resources, is providing hydrogen at lower cost. For transportation fuel cells, a key driver for energy independence, hydrogen must be cost-competitive with conventional fuels and technologies on a per-mile basis. This means that the cost of hydrogen—regardless of the production technology—must be less than $4/gallon gasoline equivalent (untaxed and dispensed). To reduce overall hydrogen cost, research is focused on improving the efficiency and lifetime of hydrogen production technologies as well as reducing the cost of capital equipment, operations, and maintenance.
Research Directions
Hydrogen production technologies are in various stages of development. Some technologies, such as steam methane reforming, are already commercial and can be used in the near-term. Others, such as solar thermochemical water-splitting, photoelectrochemical, and biological are in early stages of laboratory development and considered potential pathways for the long-term.
Related research includes developing new hydrogen delivery methods and infrastructure, improving carbon sequestration technology to ensure that coal-based hydrogen production releases almost no greenhouse gas emissions, and improving biomass growth, harvesting, and handling to reduce the cost of biomass resources used in hydrogen production.
For More Information
More information on the Fuel Cell Technologies Office is available at
http://energy.gov/eere/fuelcells/ fuel-cell-technologies-office